
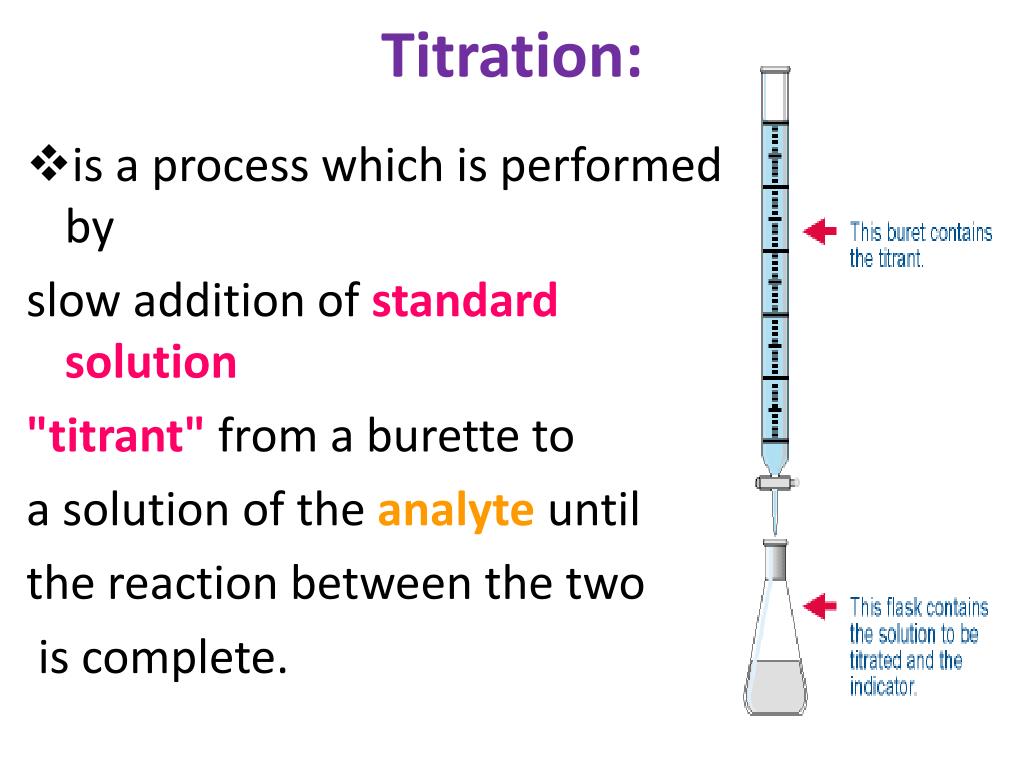
The detection at the cathode will be in the order or cations, neutral compounds, and anions. Based on the charged species and water in the system, species of all charges will reach the cathode end of the capillary since the walls of the capillary interact with the cations while water and anions will be able to travel in the middle of the column dragged along at various speeds based on size and charge. The second principle is a result of the electrophoretic force of the zeta potential and referred to as electroosmotic flow. titrate with 0.1 mol/L perchloric acid VS (potentiom- etric titration, Endpoint Detection Method in. The interaction between cations in solution and the silica lining of the column creates the zeta potential leading to migration of cations and dragging along water and neutral compounds with it towards the cathode. After attaching the cocaine using dual-thiol aptamer immobilized on the surface of MoS2AuNPs, cocaine could be detected using a cocaine aptamer with FAM. Ions will move towards their respective countercharge based on the charge and the size. Electrophoretic mobility is the result of the application of a charge to a solution of ions. determination of respective alkaloid in pharmaceutical preparations by direct potentiometry and by potentiometric titration. Two principles are mainly responsible for the flow of all charged and uncharged species toward the cathode of the system.


 0 kommentar(er)
0 kommentar(er)
